[ad_1]

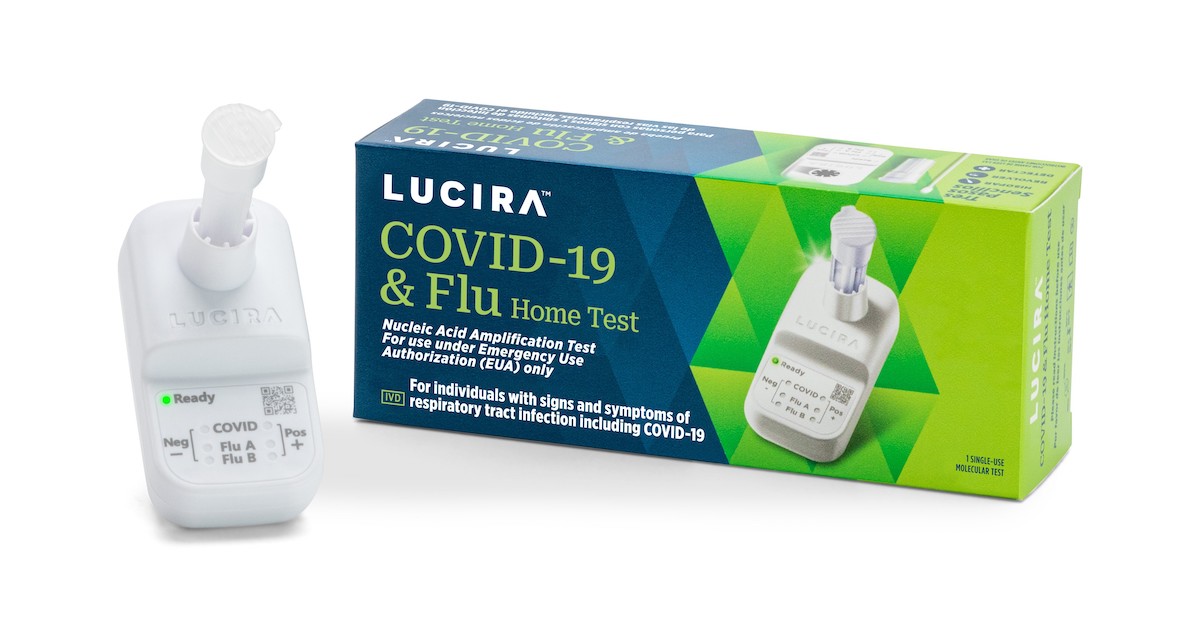
Lucira Wellbeing announced it filed for Chapter 11 individual bankruptcy past week just as the diagnostics firm received Unexpected emergency Use Authorization from the Fda for an at-home combination COVID-19 and flu take a look at.
In the company’s individual bankruptcy filing, it lists about $146 million in property and liabilities of all around $85 million. Lucira designs to use around $4.5 million money on hand to fund functions as it seeks to promote alone.
Lucira said it saw considerably less demand from customers for its residence COVID-19 test in 2022, and the FDA’s regulatory process for its combination check took for a longer period than predicted.
“We regret that we had no possibility but to file for Chapter 11 individual bankruptcy and that this happened times just before we gained regulatory authorization,” Erik Engelson, president and CEO of Lucira Health and fitness, explained in a assertion. “However we had been not able to bridge what turned a protracted authorization cycle time in just our present cash framework and it remained unclear to us when the regulatory authorization would come through, inspite of working carefully with Food and drug administration. The Lucira COVID-19 & Flu Household Examination would have been specifically valuable during the latest, severe respiratory year, and we experienced created stock for an expected autumn 2022 start.”
The combination examination marks the first authorization for an above-the-counter and at-home diagnostic that can detect and differentiate concerning the viruses that result in flu and COVID-19. It works by using a nasal swab to deliver results in about 30 minutes.
“Today’s authorization of the to start with OTC exam that can detect Influenza A and B, together with SARS-CoV-2, is a big milestone in bringing better purchaser accessibility to diagnostic assessments that can be carried out entirely at residence,” Dr. Jeff Shuren, director of the FDA’s Middle for Units and Radiological Health, explained in a assertion. “The Food and drug administration strongly supports innovation in examination improvement, and we are keen to go on advancing larger access to at-residence infectious ailment screening to very best assistance community health and fitness wants. We continue being committed to functioning with take a look at developers to assist the shared target of getting a lot more correct and reliable assessments to Us residents who need them.”
THE Greater Development
Founded in 2013, Lucira been given Crisis Use Authorization for its COVID-19 home check in late 2020. In Oct, the organization reported it would lay off 56 workforce, or about a quarter of its workforce.
Numerous electronic health and wellness tech companies have struggled monetarily after expanding and increasing significant amounts of cash in 2021. A further residence diagnostics firm, Cue Wellness, recently introduced designs to slash 26% of its workforce.
[ad_2]
Source link