[ad_1]

Medtech enterprise Masimo announced it experienced acquired Fda De Novo clearance for Opioid Halo, the company’s opioid overdose alert system.
The checking technique aims to avoid overdose by alerting relatives users or caregivers when a affected individual is enduring opioid-induced respiratory depression, or slowed or stopped respiration that can be deadly.
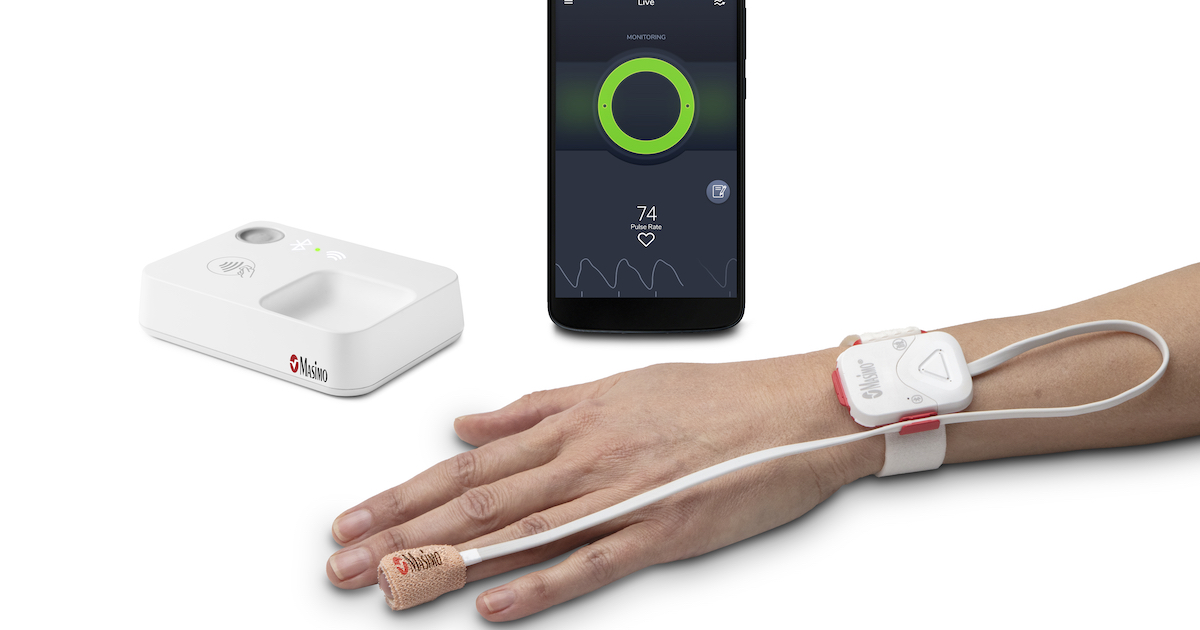
Opioid Halo involves four factors: a fingertip sensor, a reusable pulse oximeter, a household health-related hub and a smartphone app. The process delivers escalating alerts if it detects indications of respiratory melancholy, like auditory and visual alarms and automatic texts to buddies and spouse and children.
End users can also increase an optional environment that makes it possible for a service heart to make computerized wellness checks if the method detects prospective overdose, which could include dispatching crisis companies.
Masimo reported Opioid Halo is authorized for in excess of-the-counter use for people ages 15 and older. It can be also providing a prescription version of the solution.
“We are quite psyched to be in a position to offer you this resolution to our fellow Us residents and the local community heroes who are serving to to battle the opioid crisis – a disaster so devastating in its affect on the young that it has lowered in general life expectancy in the U.S.,” Joe Kiani, founder and CEO of Masimo, mentioned in a statement.
“Now, with Opioid Halo, we hope to assist make a significant big difference by delivering a considerably required resource that can enable tens of millions, regardless of whether they are getting recommended opioids or battling with illicit opioid use.”
THE More substantial Development
According to provisional facts, overdose fatalities dropped modestly in the very first nine months of 2022 soon after spiking throughout the COVID-19 pandemic. Continue to, almost 80,000 died throughout that time period of time, and artificial opioids like fentanyl are driving overdose deaths across the region.
Masimo had been doing the job on the Opioid Halo for a number of many years. The firm was bundled in the Fda Opioid Innovation Problem, introduced in 2018, that aimed to generate professional medical units, digital overall health products and solutions and diagnostics to deal with opioid abuse and misuse.
Just very last week, the Fda also accepted Narcan, a naloxone nasal spray that reverses an opioid overdose, for around-the-counter use.
Howard Rubin will give extra detail in the course of the HIMSS23 session “Rising Obtain to Treatment for Rural and Underserved Communities.” It is scheduled for Tuesday, April 18 at 3 p.m. – 4 p.m. CT at the South Creating, Level 1, place S105A.
[ad_2]
Resource website link